Introduction
Painting reconstructions usually suggest the reproductions of old master paintings which many conservation training programmes and art techniques courses include so that their students may learn about traditional painting techniques. Reconstructions, however, are also made to answer specific questions in conservation research, either in relation to analysis or to model proposed treatments. Such reconstructions are not necessarily fully accurate in terms of the materials, nor are they required to be, as toxicity issues alone impose practical limitations.
This paper explores projects where historical accuracy, as far as is possible, is the objective. Naturally there are compromises, since modern materials are unlikely to have precisely the same chemical and morphological identity as those used in the past. However, the attempt to recreate historical recipes with a high degree of accuracy provides sometimes surprising outcomes and unanticipated results
The Purpose
Art technological research (or technical art history) is gradually becoming recognised as a field in itself. Understanding how a particular work of art was created, and with what materials, can greatly enhance our interpretation of the work, its authenticity or place within the artists’ oeuvre, and its contextual relationship to contemporary works (that is, how innovative the artist was with his technique or materials). This research is highly dependent on historical documentary sources (recipes, images and observations), and on information gained through the examination of original paint samples with optical microscopy and materials analysis.1
There is, however, a gap between what can be learned from historical recipes on the one hand and from analytical results on the other. Analysis produces more and more sophisticated and reliable lists of materials present while recipes, although often fragmentary, inform us of their use. But, at a certain point, to enter the experience of the artist and his assistants, and to understand their materials, the recipes themselves must be followed. Only in this way can we investigate their validity and compare their products with what is found in original artworks.
By making reconstructions with historically appropriate materials, we can learn why materials were prepared in a certain way, what governed the artist’s choice, and how such materials behave in application, thereby gaining direct insight into workshop practices. For example, traditional mastic varnish recipes recommended ageing prior to use, for a minimum of nine months. When an aged varnish, prepared according to a traditional recipe, was compared with one freshly made (following the same recipe), the older varnish was silkier, brushed out better and allowed a longer working time before setting – features that could not be anticipated through chemical analyses alone (Carlyle 2005). Similarly, following a contemporary recipe for an artists’ medium demonstrated that brushwork and surface features observed on actual paintings were characteristic of the medium used (Townsend et al 2005). Once recreated, the products of old recipes can function as reference samples for comparison with what we see and find through analyses, and to assist the interpretation of analytical results (van den Berg et al. 1999; van der Doelen 1999; van den Berg 2002).
To evaluate change and when it occurred in the course of a painting’s history, we need to know how painters’ materials behave over time. Certain paint defects may be very visible now, but why and when they developed is not clear. How did a painting appear and behave when the paint was fresh? Reconstructions with historically accurate materials can be designed to answer these questions (van den Berg et al. 2005).
The sources
The recipes used for these reconstructions must be representative. Identifying and locating (often rare) documentary sources and understanding their provenance and bibliographic context is the first step; this is followed by analysis and interpretation of their technical information. As instructions are often incomplete, a single recipe can usually only be understood in relation to additional information from associated recipes.
Following the trail of multiple editions can establish the introduction of new materials or the passing of previously popular materials. Databases can be extremely effective for both recipe collection and analysis since they allow a series of chronological searches according to materials. As part of the HART project, a database of recipes and observations focusing on three main subject areas – lead white pigment manufacture and use, ground or preparation layers and oil processing and driers – was created from seventeenth–nineteenth-century sources in Britain, the Netherlands, France, Germany, Italy and Spain (Witlox and Carlyle 2005). A further innovative page-image based database on oil painting recipes in the nineteenth-century archive of Winsor & Newton has been developed within the De Mayerne Programme (Clarke and Carlyle 2005a, 2005b).
For effective analysis, recipe databases require continual refinement. Early tests of the report function in the HART recipe database, well before all the information was entered, revealed the need for new fields so that information could be properly accessed. For example, in the section on grounds, additional fields were necessary to identify the number of application layers and the materials used. This allowed reports to be generated regarding the use of single and double grounds, and to isolate what materials were associated with each layer.
Immediately a fundamental issue with comparing recipes and microscopic cross-sections of ground layers became apparent. A cross-section of a ground reconstruction showed that not all of the individual applications of materials were visible: separate layers were obvious only when a change in formulation in a given application layer had occurred. Therefore it was necessary to distinguish between ‘layers’ (of different formulations) and ‘applications’ (of several layers with the same composition).
The work with recipe databases has demonstrated the importance of anticipating how the information will be used and the need for continual search refinements. Flexible software enables researchers to cope with large amounts of information by using the database itself to do the work. For this function it must be possible to generate new fields at any point during data entry or analysis.
Sourcing historically appropriate materials
Once representative recipes have been identified, the next step is sourcing historically appropriate materials. How far this goes is dependent on the context of the recipe and on practical considerations, as the following examples will demonstrate.
In previous work on flour-paste ground reconstructions carried out at the Canadian Conservation Institute (CCI), one nineteenth-century recipe called for ‘calcined sheep’s trotters’. How important was it to use the foot bones from sheep? The only way to find out was to explore these bones quite literally.
Sheep’s feet were sourced from an abattoir. After lengthy boiling, the result was a collection of very small (1–3 cm) porous bones – easy to break and quick to calcine in comparison with the significantly more dense leg bones. After calcining, feet bones were relatively easy to powder and then grind, compared again with leg bones. Aside from relative ease of use, economy probably played a role, since this part of the animal was useful only for collagenous material (for example, gelatine or glue) rather than meat, and it is likely that such bones were available very cheaply from those who rendered animal carcasses.
It is probable that the particle size of calcined sheep’s bones ground using old methods is larger and more varied than in modern industrially prepared bone meal, so that differences in the mechanical behaviour of grounds made with the ‘authentic’ material may be expected. Historical accuracy begins to break down when we consider the breed and diet of modern sheep versus those in early nineteenth-century Britain where the recipe originates. Although perhaps not relevant to this study, it is conceivable that such differences could be important, for example by influencing the performance of modern eggs in egg tempera recipes as against those available in past centuries (Phenix 1997).
The flour source was also important to consider. Modern industrially prepared flours do not approximate those available in the past, and the proportion of gluten (the sticky substance in flour) would be different according to crop densities and plant type. Fortunately, an expert on historic cultivars supplied flour milled from the direct descendents of British nineteenth-century plants grown in similar crop densities to those of the past.2
The HART project has had less success with attempts to locate historically accurate linseeds for oil processing and paint preparations. However, a compromise has been reached: all reconstructions are prepared from oil extracted in the laboratory from the same seed lot, with seeds that are organically grown from flax plants intended for producing fabric. Modern plants for oil production have been modified, in some cases to produce significantly higher oil yields or to reduce the alpha-linolenic content of the oil.3
Lead white pigment
One of the principal areas of research for the HART project is lead white pigment and its possible role in lead soap aggregation, a disfiguring paint defect identified on a wide variety of oil paintings from as early as the fifteenth century through to the twentieth century (Boon et al. 2002; Noble 2003). Scanning electron microscope backscattered electron (SEM-BSE) images of oil paint cross-sections prepared with modern lead white pigment (fig.1a) compared with cross-sections from historical paintings (fig.1b) immediately reveal that the modern material bears no resemblance to that used in the past. A comparison between a modern and traditional pigment without binding medium (figs.2 a and b) quickly reveals that the crystal structure is significantly different, no doubt due to its method of manufacture. Prior to the twentieth century, the preferred method, known as the Dutch stack process, was to corrode lead metal slowly in the presence of acetic acid using either horse dung or spent tanning bark as a source of heat and carbon dioxide.
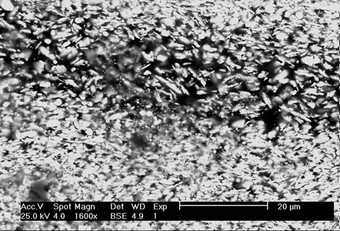
Fig.1a
SEM-BSE of paint: (a) cross-section of modern lead white (Kremer Pigmente) in linseed oil
Cross-sections made by Petria Noble, Conservation Department, Royal Picture Gallery Mauritshuis
Images taken by Annelies van Loon, FOM-AMOLF
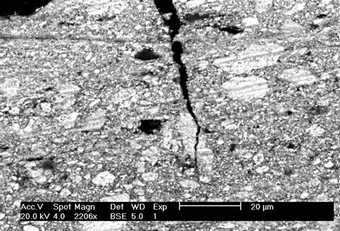
Fig.1b
SEM-BSE of paint: (b) lead white paint from white highlight in Rembrandt van Rijn, Susanna 1636 Mauritshuis inv. no. 147
Cross-sections made by Petria Noble, Conservation Department, Royal Picture Gallery Mauritshuis
Images taken by Annelies van Loon, FOM-AMOLF

Fig.2a
SEM-BSE of pigments: (a) modern lead white pigment (Kremer Pigmente)
Images taken by Katrien Keune, FOM-AMOLF

Fig.2b
SEM-BSE of pigments: (b) lead white pigment dated 1703-04, Vigani Collection, Queen’s College, University of Cambridge (Wagner 2005)
Images taken by Katrien Keune, FOM-AMOLF

Fig.2c
SEM-BSE of pigments: (c) Dutch process stack method, modern reproduction (Jef Seynaeve)
Images taken by Katrien Keune, FOM-AMOLF
Crystal morphology in the lead white pigment will probably be different according to the speed of manufacture or the corrosion products created. Within the Dutch stack process, crystal structure may also be influenced by changes in raw materials. In an early twentieth-century source, it was noted that minor amounts of hydrogen sulphide from horse dung produced traces of lead sulphide.4 Although replacing horse dung with spent tanners’ bark eliminated this ingredient, it meant that the whole process took much longer: six weeks with horse dung compared with two-to-three months with spent tanners’ bark (Petit 1907, p.16).
Dutch stack process lead white is no longer made on an industrial scale. Fortunately, a paintings conservator and painter in Belgium was making small amounts for his own use (using horse dung) and was willing to supply the HART project with enough for our reconstructions.5 A comparison between this supply (fig.2c) and eighteenth-century lead white (fig.2b) confirms that morphologically at least, the materials are very similar.
Cross-sections of oil paint made with traditional lead white (fig.3a) consistently show a large range of particle sizes: tiny particles (0.5 µm) are intermixed with very large pieces (20- 40 µm). This is apparently a feature of Dutch stack process lead white, as the same range is seen with cross-sections from our hand-ground paint made with the stack process reconstruction lead white (fig.3b).
Fig.3a
SEM-BSE of paint: (a) ground from Portrait of a Woman, Van Gogh Museum (Vincent Van Gogh Foundation inv. no. F215c), March–June 1886
Images taken by Kees Mensch, Shell Analytical Services

Fig.3b
SEM-BSE of paint: (b) Dutch process stack method, modern reproduction (Jef Seynaeve) in linseed oil
Images taken by Kees Mensch, Shell Analytical Services
Having raw lead white, freshly corroded, made it possible to study unwashed and washed samples to determine whether a lack of thorough washing (traditionally carried out to remove excess lead acetate) would encourage lead soap formation and aggregation. Although it is not always possible to reproduce past materials accurately (for example, differences in past trace materials in the metallic lead or the diet of the horses), this work clearly demonstrates that preparation methods that mimic essential conditions used in the past can at least approximate traditional materials – certainly much more closely than modern equivalents.
Workshop practice
Carrying out reconstructions with old recipes provides surprising insights into workshop practices, which would have been obvious to artists and their studio assistants in the past. In an earlier study on the influence of oil processing methods, it was quickly determined that the way an oil is treated prior to use, and the pigment used, has a profound influence on the flow properties of the paint (rheology). Lead white in particular ranged from unmanageably stringy in oil heated to 300°C through to fluffy and buttery in freshly extracted untreated oil (Carlyle 2001a). Similarly, the HART project’s study of water-washing oil with and without salt (called for in early recipes), demonstrated that the drying time for the oil was strongly influenced by whether it was stored for long periods prior to use (without washing), was freshly extracted or was washed either with or without salt. Untreated oil extracted three years previously was more clear and dried significantly more quickly than freshly extracted oil from the same seed lot, confirming reports in artists’ manuals that simply keeping oil for long periods resulted in a superior product (Carlyle 2001b, p.32).
Oil processing with and without heat and lead-based driers was found not only to influence the handling qualities of the paint but also its colour. Lead white paint made with oil treated with litharge (lead (II) oxide) or lead acetate was more yellow than that made with oil heated to 300°C without driers (Carlyle et al. 2002). This may well relate to reports in historical sources of the ‘darkening’ effect of the oil on the paint (Carlyle 2001b, pp.23–4, 257–60). Oil painters in the past repeatedly complained of a ‘greasy layer’, which appeared on the surface of their dried oil paint and resisted further applications of paint. This was not confined to nineteenth-century painters (Carlyle 2001b, pp.205–7, 229): the addition of spike oil to prevent an oily skin on the surface of blue paint is recommended in the de Mayerne Manuscript (1620–1644: folio 11r). This phenomenon has been observed with HART paint samples and is thought to be related to hand-grinding (which requires more oil than machine-grinding), and to the lack of suspension agents (such as stearates or hydrogenated castor oil), commonly used in modern paints. As we get closer to approximating historical paint recipes, we encounter technical problems familiar to painters in the past.
Cross-contamination of sample
Throughout the HART project, every effort was made to avoid contaminating paint samples with pigments or materials being prepared at the same time. One workshop involved eleven differently prepared lake pigments with up to six people grinding paint simultaneously, using a variety of binders. Brushes, palette knives, glass mullers and slabs were all clearly labelled so that those used for cochineal lakes, for example, were not then used for madders etc. The care and planning necessary to avoid mixing materials made it obvious that stray pigments could easily be introduced unintentionally in an artist’s studio or workshop. The difficulty of adequately cleaning brushes and grinding equipment even with modern methods made it clear that artists, too, struggled with this issue over the centuries.
Substrates and application methods
Aside from striving for historical accuracy in the materials chosen and the preparation methods, the choice of appropriate substrate is essential. Oil paint will dry differently according to the substrate’s porosity or lack of it. Therefore a single paint in the HART project is applied to a range of substrates including traditionally prepared canvas (lead white in oil ground or flour-paste ground), modern artists’ boards (titanium white in acrylic priming), polyester film, ceramic tiles and glass slides. SEM-BSE images of red lead show clearly that oil separation occurs only along the top when the sample is applied to canvas, but can be seen in both top and bottom surfaces where it has been applied to glass (figs.4 a and b). Non-porous surfaces are especially problematic for oil paint, sometimes resulting in severe wrinkling and higher amounts of oil rising to the surface than when more porous substrates are used.

Fig.4a
SEM-BSE of red lead (Fisher Scientific) paint showing (a) oil rich layer at the surface, paint applied over glue sized canvas
Cross-sections prepared by Kathrin Pilz. Images by Kees Mensch, Shell Analytical Services

Fig.4b
SEM-BSE of red lead (Fisher Scientific) paint showing (b) oil rich layers at top and bottom of sample, paint applied on glass
Cross-sections prepared by Kathrin Pilz. Images by Kees Mensch, Shell Analytical Services
These observations may be extrapolated to actual paintings where porosity (or lack of it) in the ground or paint layers will have influenced the way subsequent paint dries. It is important to note that ‘paint porosity’ or the propensity for oil absorption in a given paint layer will also be dependent on the pre-processing and state of polymerisation (or drying) of the oil binder when subsequent layers are applied.6
Application methods also play a role in the formation of the paint film. In the HART project, paint samples are applied with a brush or palette knife, and as draw-downs or cast films of uniform applied thickness. The use of the palette knife often results in a layer of oil at the surface of the paint which is not seen in brushed-out samples. Indeed, artists were warned, ‘that colours should not be mixed too much on the palette as the oil will then rise to the surface’ (Mérimée 1839, p.337).
Scaling down recipes
Following artists’ recipes prior to the Industrial Revolution offers a higher likelihood of scale and workshop practice (such as hand-grinding) being similar enough. However, scaling down to laboratory conditions recipes and procedures meant to be carried out in a factory is problematic. How can we compare a 200 ml of oil boiled in a laboratory beaker for one hour with twenty gallons in a copper vessel boiled for eight days? Machine-grinding can be replicated to some degree with a laboratory-sized triple-roller mill, but how well does that actually compare with industrial quantities and with the various roller mill designs used in the past, or even the effect of a granite roller versus steel?7
Reconstructions based on cross-sections from actual paintings
One of the aims of the HART project is to reconstruct grounds based on analyses of selected paintings by Vincent van Gogh to compare how his fresh paint would have behaved on the different types of ground seen in his collected works. This, more than any other project, points out the limitations of instrumental analyses for providing information on the precise nature of an artist’s materials. Extensive SEM and energy-dispersive X-ray (EDX) analysis combined with microscopic examination of paint and ground cross-sections have identified the materials present in a broad range of his grounds.8 However, for reconstructions, identifying what is there is not enough: in order to prepare similar grounds we must know the proportion and characteristics of the ingredients present.

Fig.5a
SEM-BSE of paint reconstructions with (a) 15% unrefined barium sulphate (Sachtleben) and 85% modern lead white (Kremer Pigmente) in linseed oil
Cross-sections prepared by Kathrin Pilz. Images by Kees Mensch, Shell Analytical Services
Initial investigations with EDX mapping9 were undertaken with a series of reconstructions based on stepped proportions of additives (barium sulphate or chalk) found in the grounds. Results with oil paint cross-sections of barium sulphate in different concentrations with modern lead white were encouraging, showing good correlation in weight concentration and atomic concentration at proportions up to 50%. But at 75% barium sulphate to lead white, the correlation was poor. Visual comparisons were straightforward as the unrefined barium sulphate (which approximates in shape and particle size to that used in some van Gogh grounds) was easy to distinguish (fig.5a, compare with fig.3a). Therefore, a good guess was possible when comparing the stepped proportions with one another, even up to 75%. But with chalk and lead white in stepped proportions, BSE images do not provide adequate information for a visual distinction. In these samples, the quantification program was essential. Again, it showed good correlation within ± 8 percentage points.

Fig.5b
SEM-BSE of paint reconstructions with (b) 50% unrefined chalk (Omya) and Dutch process lead white (Jef Seynaeve)
Cross-sections prepared by Kathrin Pilz. Images by Kees Mensch, Shell Analytical Services
However, a sample prepared with the traditional stack process lead white reconstruction and 50% chalk (fig.5b) has not resulted in an accurate prediction with the software: chalk appears in excess. The reason for this discrepancy is not currently known, but points out the difficulty of relying on EDX quantification software and the need for careful calibration against known standards.
A further difficulty surrounds the precise identification of the chalk or calcium carbonate. Although some van Gogh samples show characteristic Foraminifera or coccoliths associated with marine deposits, others are less clear. Given the wide variety of calcium carbonates available and the difference in their characteristics and working qualities, this information is important.
One obvious way around the problems of evaluating accurate proportions of ingredients, and the exact identity of a material, is to make a series of reconstructions where these variables are explored using different proportions and sources of materials. The advantage of this methodology is that cross-sections from the resulting reconstructions can provide reference sets for future investigations and for comparison with cross-sections from historical paintings.
Future reconstructions within the HART project
Future plans include reconstructions of paint composites where paints made with different oils (for example, linseed oil or poppy oil) are layered with intermediate layers between (such as mastic varnish, oiling-out layers or glue). How oil processing and the degree of drying in a given paint influences its propensity to absorb binder from other layers will be studied10 as well as the impact of mixing linseed and poppy oils in the production of a single paint.
The future of the reconstructions
So-called ‘historically accurate’ reconstructions (as understood within the context described in this paper) will prove valuable to future researchers, possibly in ways never anticipated by the current makers. Therefore, it is essential that samples are properly documented with as much precision as possible and that this documentation is not separated from the samples themselves. Furthermore, documentation (including labelling) must be produced to archival standards. Storage and display of the samples requires careful planning for the future. Although appearing ‘dry’, oil paint samples may be susceptible to damage if they are stacked. Therefore, surfaces must be protected against contact with space for air circulation maintained between each sample. Surrounding storage materials must be relatively inert, not contributing to degradation or contamination by off-gassing or migration of modern materials such as plasticisers.
The institution where the samples are housed must make a commitment to their continued care. All too often, past reconstructions by our predecessors have been simply discarded because the documentation was no longer available or their value was not recognised.
Sets of samples, like those from the HART project, where oil from the same seed lot has been used throughout with a limited number of well-characterised pigments, will provide future researchers with reference samples for many years to come – not to mention the added value of having naturally aged samples to study.
