The use of oil-based paints has been the main process for painting since the middle-ages and remains ever popular today. As there are many examples of apparently well-preserved early oil paintings in museum collections, one might assume that oil painting is a well understood and time-tested painting technique. However some oil paintings made in the twentieth and twenty-first centuries, and particularly those intentionally left unvarnished, are posing a series of unexpected and somewhat under-explored conservation challenges. One such challenge is that many twentieth century oil paintings are exhibiting water sensitivity.1

Joan Mitchell
Chord II (1986)
Tate
In water sensitive paintings when a dampened cotton swab (for example) is gently rolled over the paint surface, some pigment and binder are removed, which can cause concern and impacts established conservation practices for modern and contemporary oil paintings.
Paintings are prone to picking up surface dirt during display and over time, which can alter the appearance of the colours by reducing brightness and saturation, and changing surface gloss. Significant dirt build-up can also detract from the artists’ original intention and even promote certain types of degradation. For these reasons surface dirt removal (also known as surface cleaning) is often considered a necessary step to recover the brightness of a work and to minimise any detrimental effects on the paint layers underneath.
Unfortunately many of the most effective surface cleaning agents are created using water, which poses a great challenge for safe use on very water sensitive paintings. In recent years research efforts 2 3 have contributed to our understanding of modern oil paint chemistry and have identified some of the factors that are involved in causing water sensitivity and other degradation phenomena noted on paintings. Concurrent research strands4 are now beginning to contribute options for surface cleaning that are better suited to these types of sensitive paint surfaces such as rigid gels, emulsifier gels and microemulsions, in addition to the increasingly skilful use of sponges and temporary barriers which help to slow down the action of water-based cleaning agents such as non-polar solvents and/or tissue(s).
Understanding the causes of water sensitivity in modern and contemporary oil paintings also requires us to understand more about oil paint formulations and paint manufacturing processes. Artist quality oil paints generally contain an oil binder (drying or semi-drying oils such as linseed, poppy or safflower oil), mixed with pigments, extenders (e.g. chalk, barium sulphate), and other additives that change the rheology of the paint (metal soaps), make the paint stable, and make the paint dry faster (driers). Oil paints dry upon exposure to air and light because the oil binder undergoes chemical reactions that result in cross-linking (polymerisation) which changes the paint from a liquid, to a rubbery solid. However even after the paint feels dry to the touch, there are many complex chemical reactions that continue to subtly change the composition of the paint over time, which are influenced by the type of pigment present among other factors, such as the amount of oil present, the presence of specific additives and exposure of paint films to UV light, pollutants, temperature and humidity.

Fig.2
An example of a Winsor & Newton Artists’ (W&N) Oil Colour swatch dating to 1957, shown in tungsten and UV light. The swatches were made by W&N as part of quality control procedures. Paint samples were applied to a primed canvas and the name of the paint written underneath along with the drying time. A collection of swatches was donated by ColArt UK to Tate for research purposes in 2011.
Photo © Judith Lee
Recent research carried out at Tate and the Courtauld Institute of Art, London through CMOP5 and as part of an AHRC-funded collaborative doctoral award has demonstrated that a group of water sensitive Winsor & Newton (W&N) oil paint swatches and samples removed from a series of twentieth century oil paintings all contain high levels of polar materials (molecules with oxygen incorporated into them such as alcohols and acids) caused through degradation reactions that contribute to the water sensitivity of the paint.6 Recent chemical analysis of W&N paint swatches carried out at the University of Pisa (another CMOP project partner) also revealed the use of castor wax in water sensitive phthalocyanine green paints.
Another cause relates to the degradation of extender pigments (generally white solids) added to modern oil paint formulations. For example, it has been shown that paints containing magnesium carbonate extender can degrade upon exposure to environments rich in sulphur dioxide, which causes the eventual formation of water-soluble magnesium sulphate salts (Epsom salts)7 8 on the surface of affected paints. Other factors such as exposure to environmental conditions such as high humidity, in addition to how the artist might modify and use their paints will also play a part. To date, we have investigated a series of tiny paint samples removed from 24 oil paintings in Tate’s collection, which is beginning to inform our understanding of how the paintings were made, how they have aged, and to identify potential preservation challenges. The study of paintings in Tate’s collection has also enabled the identification of visual indicators for different classes of water sensitivity, which will aid conservators in assessing the condition of modern oil paintings.
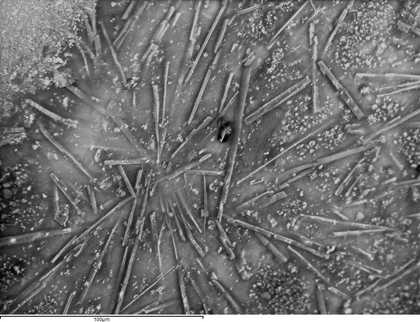
Fig.3
A backscattered scanning electron microscope image of a paint sample taken from a W&N cadmium lemon swatch dating to 1963. The image shows the characteristic rod shaped crystals of magnesium sulphate heptahydrate (Epsom salts) that are one known cause of water sensitivity.
Photo © Judith Lee
One of the other CMOP project aims is to develop and/or modify and evaluate surface cleaning materials and methods for use on water sensitive and other modern oil paints. As part of this project, another recent collaboration between Tate and the Courtauld Institute of Art, included a post-graduate student research project where the effects of surface cleaning systems that were developed for acrylic paints where explored for use on modern oil paints. This involved making test paint films using Winsor & Newton Artists’ Oil Colour paints that had been applied directly to primed canvas and light-aged to approximate the effects of around 50 years of natural ageing. An artificial soil was applied to some of the test paint surfaces which, together with an archival test painting dating from the 1970s, were used to evaluate the surface cleaning options.
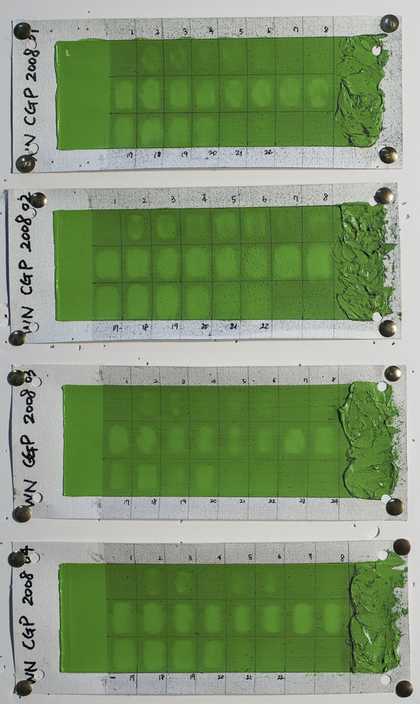
Fig.4
Tubes of artists’ oil paint that were made by W&N were used to produce test paint films for evaluating cleaning systems. In this example ‘cadmium green pale’ dating to 2008 was used. A draw-down bar was used to create samples of uniform thickness, and a brush was used to create an area of impasto. Paints were applied to acrylic and oil-primed canvases. After drying and light ageing, an artificial soil was applied, and different cleaning systems were tested for efficacy and any effects on the paint films.
Photo © Jae Youn Chung
While none of the materials tested proved ideal (which is rare in any case!) for these paint surfaces (sometimes cleaning was ineffective, or undesirable levels of pigment pickup was observed); however adjusting the pH and conductivity (free ion content) of water-based cleaning systems, as well as the use of silicone-based emulsifiers recently introduced into conservation by Richard Wolbers, showed some promise by offering the ability to remove the soiling while causing minimal change to the underlying paint film properties.
In the coming months these materials will be investigated further, through more systematic testing that will explore other methods of application, further modification to the systems that proved promising and the incorporation of other cleaning options used by conservators. To support this research, in spring 2017, a test sample paint-making workshop is planned to produce model paint films for further testing and study, hosted by one of the CMOP project partners RCE (Cultural Heritage Agency of the Netherlands) and the Rijksmuseum in Amsterdam; do look out for some pictures in our next update.
Judith Lee and Bronwyn Ormsby, Tate
March 2017
